π-Conjugated Inorganic—Organic Hybrid Polymers
Research
π-Conjugated Inorganic—Organic Hybrid Polymers
π-Conjugated polymers with semiconducting properties have attracted considerable attention owing to their use as cost-effective, lightweight materials for applications in electronic and optoelectronic devices such as organic light-emitting diodes (OLEDs/PLEDs), organic field-effect transistors (OFETs), and organic photovoltaic cells (OPV), or as sensory or imaging materials. Recent advances have shown that the incorporation of inorganic elements into conjugated polymers leads to novel hybrid materials with intriguing properties and functions that cannot be achieved with purely organic compounds.
Particularly, the main chain functionalization with boron atoms opens up new possibilities. Extension of the π-conjugation via the vacant p orbital on boron results in the formation of strongly electron-accepting materials which can be reductively doped, thus yielding favorable electron-transport (n-channel semiconducting) abilities. Such species are often highly photo- and/or electroluminescent, and the Lewis acidity of trivalent boron additionally offers the opportunity to generate polymeric sensors for the specific recognition of physiologically relevant anions (e. g. fluoride, cyanide) or amines.
Regioregular Poly(p-phenylene iminoborane): A StrictlyAlternating BN-Isostere of Poly(p-phenylene vinylene) with Stimuli-Responsive Behavior
An inorganic poly(p-phenylene vinylene) (PPV) analogue with strictly alternating BN units along the chain was prepared by an AB-polymerization process. The polymer and monodisperse oligomers show emission from locally excited (LE) or twisted intramolecular charge transfer (TICT) states, respectively, which can be addressed by various stimuli, giving rise to solvatochromic, thermochromic, and reversible mechanochromic behavior.
Poly(arylene iminoborane)s, Analogues of Poly(arylene vinylene) with a BN-Doped Backbone: A Comprehensive Study
M. Maier, J. Chorbacher, A. Hellinger, J. Klopf, J. Günther, H. Helten*, Poly(arylene iminoborane)s, Analogues of Poly(arylene vinylene) with a BN-Doped Backbone: A Comprehensive Study, Chem. Eur. J. 2023, e202302767.

Polymers, copolymers, and oligomers from combinations of backbone-BN-doped poly(p-phenylene vinylene) (PPV) and poly(thiophene vinylene) (PTV) with tailored properties have been prepared by a modular approach. The π-conjugated donor–acceptor-type backbone structure gives rise to efficient solid-state emission and aggregation-induced emission (AIE) behavior.
Poly(thiophene iminoborane): A Poly(thiophene vinylene)(PTV) Analogue with a Fully B=N-Doped Backbone
J. Chorbacher, M. Maier, J. Klopf, M. Fest, H. Helten,* Poly(thiophene iminoborane): A Poly(thiophene vinylene) (PTV) Analogue with a Fully B=N‐Doped Backbone, Macromol. Rapid Commun. 2023, 2300278 [DOI]

Poly(thiophene iminoborane) (BN-PTV) is a BN analogue of poly(thiophene vinylene) (PTV). The first derivative of this class of novel hybrid polymers has been prepared by facile Si/B exchange polycondensation. The series of monodisperse thiophene iminoborane oligomers and the polymer reveals extended conjugation over the polymer backbone, including the B=N moieties.
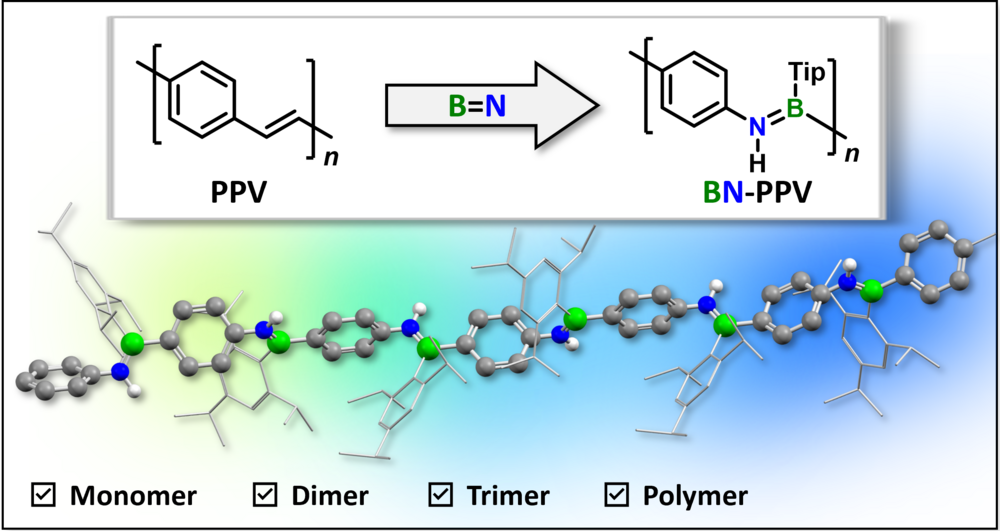
Poly(p-phenylene iminoborane): A Boron—Nitrogen Analogue of Poly(p-phenylene vinylene) (PPV)
A BN analogue of the well-known semiconducting organic polymer poly(p-phenylene vinylene) (PPV) has been synthesized via a facile Si/B exchange polycondensation approach. The novel inorganic—organic hybrid polymer and monodisperse oligomers of this type show π-conjugation across the B=N units along the backbone and extension of the conjugation path with increasing chain length.
Dehydrocoupling and Silazane Cleavage Routes to Organic—Inorganic Hybrid Polymers with NBN Units in the Main Chain
T. Lorenz, A. Lik, F. A. Plamper, H. Helten, Angew. Chem. Int. Ed. 2016, 55, 7236—7241 [DOI]; Angew. Chem. 2016, 128, 7352—7357 [DOI].
Highlighted in: "A New Route to Polymers with NBN Backbone", T. M. Swager, Q. Zhang, Synfacts 2016, 12, 0691 [DOI].
Highlighted in: "Trendbericht Makromolekulare Chemie 2016", M. Sommer, F. Wurm, Nachr. Chem. 2017, 65, 348—358 [DOI].

Polymerization through B—N bond formation provided facile access to the first derivatives of a new class of inorganic—organic hybrid polymers having diaminoborane functional groups incorporated into a conjugated polymer backbone. The new materials can be crosslinked via ZrIV centers, thus, demonstrating their applicability as macromolecular poly-ligand systems.
Catalytic B—C Coupling by Si/B Exchange: A Versatile Route to π-Conjugated Organo-borane Molecules, Oligomers, and Polymers
A. Lik, L. Fritze, L. Müller, H. Helten, J. Am. Chem. Soc. 2017, 139, 5692—5695 [DOI].

Using a novel, highly efficient and environmentally benign B—C bond formation method based on a catalytic Si/B exchange reaction, we have prepared a series of arylborane molecules, oligomers, and polymers. Significantly, this method offers the possibility to apply AB type polycondensations for the synthesis of conjugated organoborane polymers via B—C coupling for the first time. Photophysical investigations, supported by TD-DFT calculations, reveal highly effective π-conjugation in thienyl- and furylborane species; the latter also being highly emissive.
A. Lik, S. Jenthra, L. Fritze, L. Müller, K.-N. Truong, H. Helten, Chem. Eur. J. 2018, 24, 11961—11972 [DOI] ("Very Important Paper").

Through variation of the furan-to-thiophene ratio, the photophysical properties of these materials are effectively modulated. They are furthermore attractive targets for chemical sensory applications. Binding of fluoride anions is effectively signaled by an optical response.