Cancer Research: DNA Repair Mechanism Further Elucidated
05/28/2024Researchers at the University of Würzburg, led by Caroline Kisker in cooperation with Claudia Höbartner, discovered how the protein XPD detects a severe DNA damage and controls its repair.
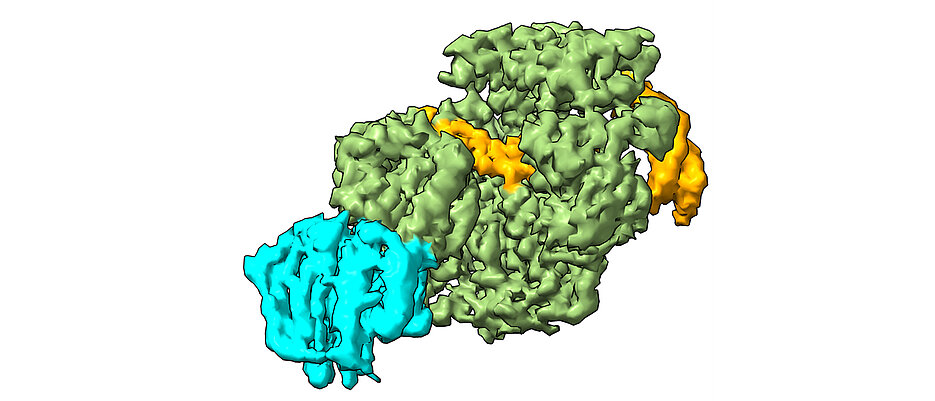
The XPD protein is a central component of our body's own 'DNA repair team', known as nucleotide excision repair (NER). Like a sniffer dog, NER detects marked areas of damage, tracks down the damaged DNA and recruits other repair proteins to cut out and replace the defective sections. In healthy people, for example, XPD prevents the development of skin cancer by detecting and repairing UV-damaged DNA.
A team of researchers at the University of Würzburg (JMU) has now discovered for the first time exactly how the XPD protein is able to detect and verify the presence of a DNA damage. The team was led by the biochemist Caroline Kisker, Chair of Structural Biology at the Rudolf Virchow Centre in Würzburg, in collaboration with the chemist Claudia Höbartner from the Department of Organic Chemistry.
Study of Severe DNA Damage
The Würzburg team focused on how the XPD protein works in interstrand crosslinking – one of the most severe forms of DNA damage known. It is caused, for example, by environmental toxins and industrial chemicals. “Interstrand crosslinking causes DNA to be incorrectly copied and read during cell division”, explains Caroline Kisker. “This leads to genetic damage that can trigger cancer.”
In their study, the scientists used cryo-electron microscopy to analyse how XPD unwinds the double helix of DNA to reveal the defective sites of interstrand cross-linking, and created a model of how the damage is detected and removed. “The findings from our work provide the basis for new approaches to treating various types of cancer”, says Jochen Kuper, employee in Kisker's team. “By specifically weakening repair mechanisms such as NER in cancer cells, we could significantly increase the effectiveness of drugs.”
In further studies, the research team plans to investigate how XPD detects various other types of DNA damage. The research was funded by the German Research Foundation (DFG) and German Cancer Aid.
The Rudolf-Virchow-Centre in Würzburg
The Rudolf Virchow Centre (RVZ) for Integrative and Translational Imaging is an interdisciplinary research centre focusing on the visualisation of elementary life processes – from the sub-nano to the macro scale. As a central institution of the University of Würzburg, the Centre is currently home to 13 translational research groups and around 100 researchers investigating the molecular causes of health and disease.
About the Study
Jochen Kuper, Tamsanqa Hove, Sarah Maidl, Hermann Neitz, Florian Sauer, Maximilian Kempf, Till Schroeder, Elke Greiter, Claudia Höbartner & Caroline Kisker. „XPD stalled on crosslinked DNA provides insight into damage verification“. Nature Structural and Molecular Biology. DOI: 10.1038/s41594-024-01323-5
Contact
Prof. Dr. Caroline Kisker, Chair of Structural Biology at the Rudolf Virchow Centre - Centre for Integrative and Translational Bioimaging and Vice President for Research and Young Researchers, Tel. +49 931 31-80381, caroline.kisker@uni-wuerzburg.de
Prof. Dr. Claudia Höbartner, Chair of Organic Chemistry I, Tel. +49 931 31-89693, claudia.hoebartner@uni-wuerzburg.de


